Writing a health claim for your product can be a powerful tool for attracting customers and communicating its benefits. However, crafting a compliant health claim requires a careful understanding of regulatory guidelines to ensure accuracy and transparency.
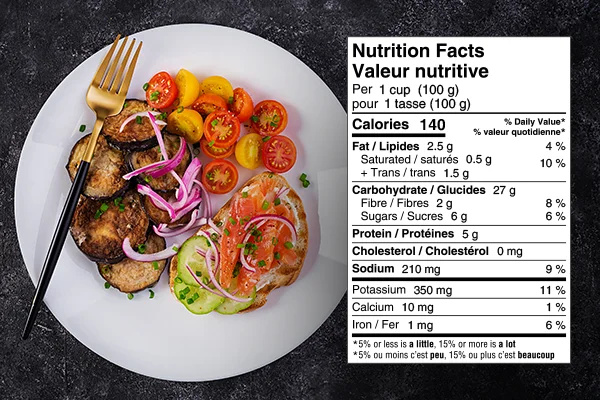
What is a Health Claim?
A health claim is a statement on food or dietary supplement labelling that suggests a relationship between a product and health benefits. The FDA, for instance, regulates health claims to ensure they are truthful and not misleading. Health claims can significantly influence consumer decisions and are a powerful marketing tool, but they must be carefully crafted and legally compliant.
Types of Health Claims | FDA
Health claims on food labels in the United States are regulated by the Food and Drug Administration and are categorized into three main types:
- Health Claims: These claims describe a relationship between a food or dietary ingredient and a reduced risk of a disease or health-related condition. Health claims must be supported by scientific evidence and can be either authorized or qualified. Authorized health claims meet a rigorous scientific standard, while some evidence supports qualified health claims but requires a disclaimer to communicate the level of scientific support.
- Nutrient Content Claims: These claims describe the level of a nutrient in a product, using terms like “low,” “high,” “free,” “reduced,” or “more.” The FDA strictly defines them and provides consumers with information about the nutritional content of a food or dietary supplement.
- Structure/Function Claims: These claims describe the role of a nutrient or dietary ingredient in supporting normal structure or function in the human body, such as “calcium builds strong bones.” Unlike health claims, they do not refer to a specific disease and are not subject to FDA pre-approval, though they must be truthful and not misleading.
Read more: What is “Daily Value” on Food Labels?
Types of Health Claims | CFIA
In Canada, the Canadian Food Inspection Agency (CFIA) recognizes several types of permissible health claims that can be used on food labels:
- Nutrient Content Claims: These claims provide information about the amount of a specific nutrient in a food product, using terms like “source of,” “high in,” or “free of.” They help consumers understand the nutritional content of a product at a glance.
- Nutrient Function Claims: These claims describe the beneficial effects of a nutrient or food component on the body’s normal functions or biological activities. For example, “Calcium helps build strong bones.” These claims do not refer to a specific disease or health condition.
- Disease Risk Reduction Claims: These claims state that consuming a particular food or nutrient can help reduce the risk of developing a specific disease or condition. These claims require substantial scientific evidence and must be pre-approved by Health Canada.
- Therapeutic Claims: These claims indicate that a food or nutrient has a role in treating or mitigating the effects of a disease or condition. They are highly regulated and require rigorous scientific validation before being approved for use on food labels.
Read more: Front-of-Package Labelling in Canada – Now Available on MenuSano
How to Submit Write a Health Claim in Canada
Submitting a health claim in Canada involves a few key steps based on the guidance document for preparing a submission for food health claims provided by the CFIA.
- Gather Scientific Evidence: Collect and evaluate research that supports the health claim you want to make. Your submission should include compelling data to support your claim. Otherwise, you risk rejection.
- Prepare a Submission: Compile your findings into a detailed submission, following the guidelines provided by Health Canada. This includes providing all relevant studies, data, and justifications.
- Submit for Review: Submit your documentation to Health Canada for evaluation. They will review the evidence and decide whether the claim meets the required standards. Health Canada will inform you by letter that the submission has been received within 15 days.
- Approval or Rejection: Health Canada will either approve the claim for use on food labels or request additional information. Be ready for possible CFIA feedback; feedback could include a request for more information and/or clarification.
Successfully navigating the health claim approval process requires thorough preparation and strict compliance with regulatory standards. For comprehensive instructions, consult Health Canada’s Guidance Document on Preparing a Submission for Food Health Claims.
Read more: Understanding Added Sugars on the Nutrition Facts Label
How to Submit and Write a Health Claim in the US
To submit health claims in the U.S., you must follow the guidelines set by the FDA. The process includes:
- Gathering Scientific Evidence: Collect comprehensive scientific research supporting your claim before submitting a qualified health claim. This includes studies, clinical trials, and relevant data demonstrating the link between the food component and the claimed health benefit. The evidence should be well-documented and credible to meet FDA standards.
- Preparing a Petition: Once the evidence is gathered, draft a petition to the FDA. This document should include a detailed explanation of the health claim, a summary of the supporting evidence, and the proposed wording. Ensure that the petition adheres to the FDA’s guidelines for content and format.
- Submitting to the FDA: Submit your petition to the FDA for review. The agency will assess the quality and validity of the evidence presented. During this review, the FDA determines whether the scientific support is sufficient to allow the health claim on food labels as an authorized or qualified claim.
- FDA Review: The FDA reviews your submission to determine if it meets the criteria for either an authorized or qualified health claim. After reviewing the petition, the FDA will issue a decision. If the claim is approved, it can be used on food packaging with any necessary disclaimers. If additional information is needed or the claim is rejected, the FDA will provide feedback on what is required to meet approval standards.
We encourage you to refer to the FDA’s resources on health claims submissions for detailed guidance.
Read more: How to Use Nutrition Analysis and Health Claims for Marketing
Get Help With Your Health Claims
Navigating the complexities of health claims can be daunting, but MenuSano is here to simplify the process. Our software ensures that your nutrition labels are fully compliant with the latest regulations, keeping you aligned with both the US FDA and Canada’s CFIA standards. With our easy-to-use platform, you can effortlessly update your labels to reflect the most accurate nutrient information. You also ensure your claims are valid, transparent, and trustworthy. Trust MenuSano to streamline your health claims so you can focus on what matters most, providing valuable, reliable information to your customers. You can start a free trial or schedule a demo.
Complying with government health claim regulations is crucial for food businesses, ensuring that the information provided to consumers is accurate, reliable, and legally sound. Adhering to these regulations helps build customer trust by guaranteeing that health claims are supported by scientific evidence and meet the required standards. Compliance not only avoids potential legal issues and fines but also enhances the credibility of your brand in a competitive market. By aligning with regulatory guidelines, businesses can effectively communicate the benefits of their products, attract health-conscious consumers, and foster a reputation for transparency and integrity. Ultimately, compliance contributes to long-term success and consumer confidence in your brand.


















